Innovative Healing Solutions
Our recombinant keratin wound dressing represents the forefront of medical wound care technology. Developed with the latest biotechnological advances, these dressings are designed to significantly enhance the healing process for various types of wounds, including chronic wounds, burns, and surgical incisions.
Our recombinant keratin offers significant advantages over traditional keratin sources. Through our advanced manufacturing processes, we are able to achieve a keratin purity of 95% or higher. This exceptional level of purity makes our keratin ideal for medical and healthcare applications, where high-quality, safe ingredients are of the utmost importance. In contrast to keratin obtained from natural sources, our keratin is essentially free from impurities, contaminants, and other undesirable substances that can be present in animal-derived keratin. By leveraging cutting-edge technology and stringent quality control measures, we are able to deliver a keratin product that meets the most stringent regulatory standards and exceeds the purity levels achievable through conventional keratin extraction methods, allowing our clients to confidently incorporate our keratin into their products.

01
Human derived keratin gene
*SOURCE
02
Modification and optimization of chassis cells
*HOST SYSTEM
03
Synthetic biology
*CORE BIOTECH
04
Repair/Reborn/Moisturize
*CORE FUNCTIONS
05
Biological activity
*HIGHLY ACTIVE
06
Keratin of exceptional purity, over 95%
*OVER 95%
07
No immunogenicity
*ABSENT
08
No cytotoxicity
*ABSENT
Application Area

WOUND DRESSINGS
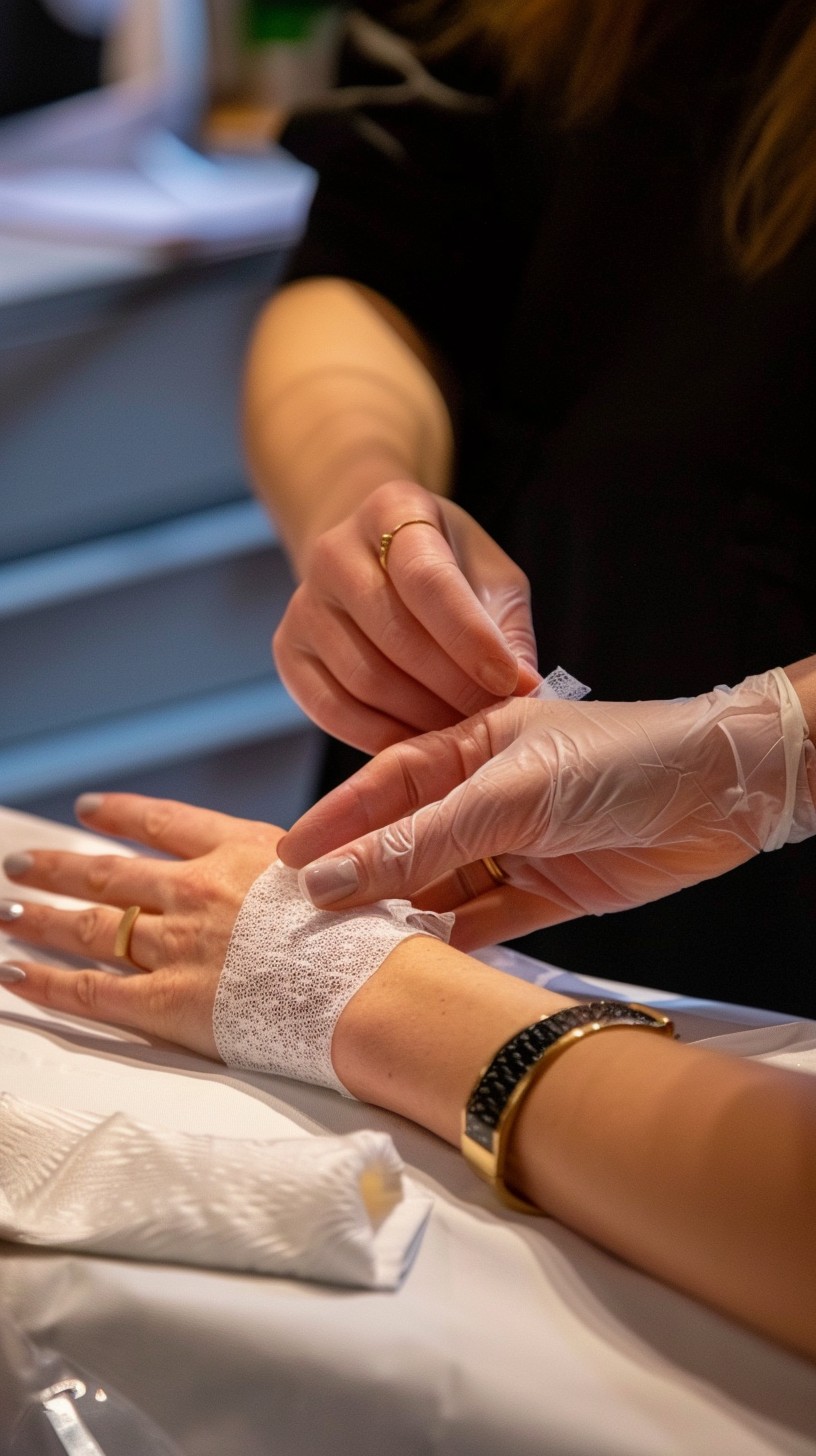
HEMOSTATIC GAUZE
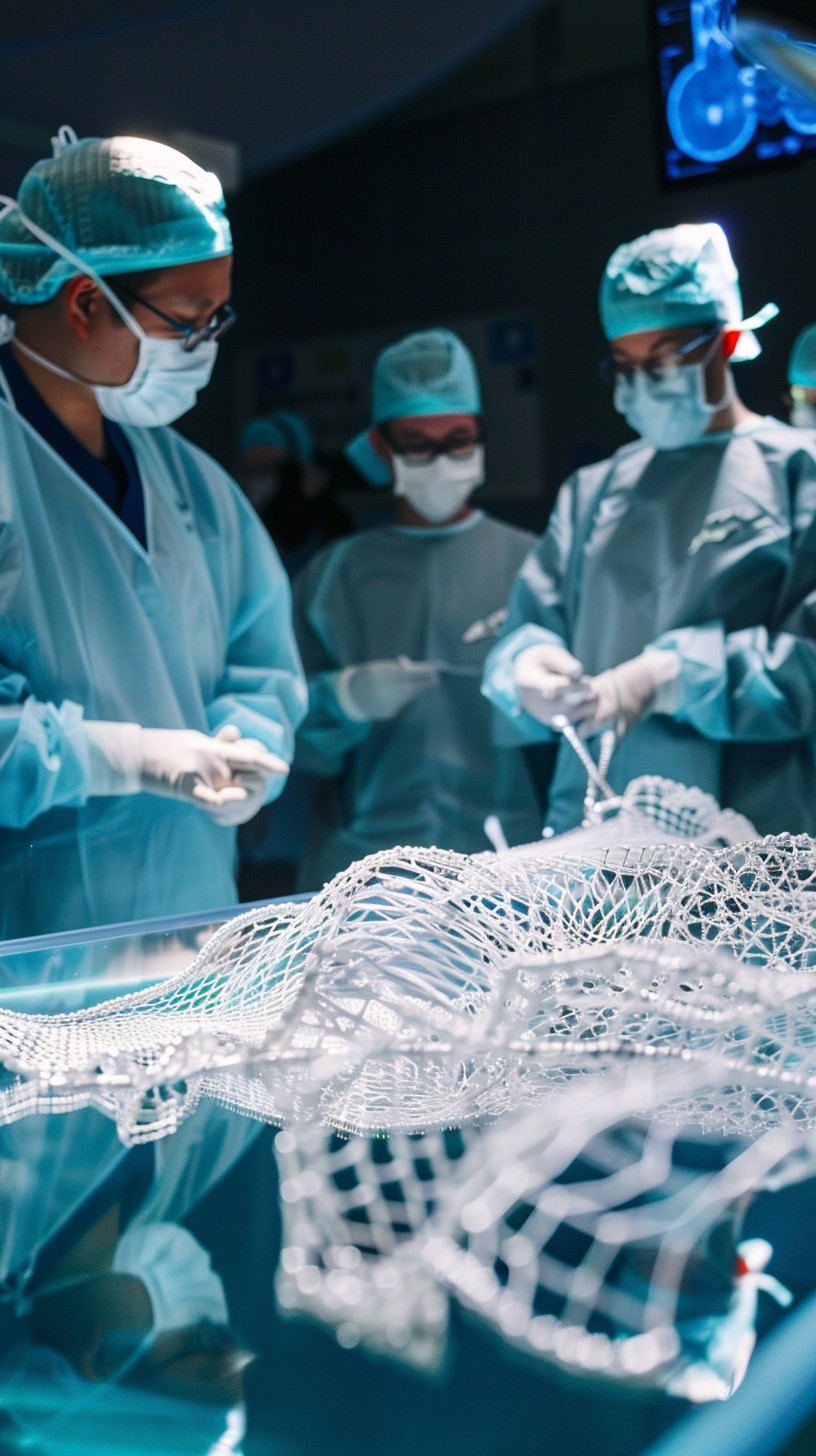
BIOMEDICAL MATERIALS
Leading Technology
Pioneering Technological Breakthroughs
Leveraging iTRAQ and DNA recombination technologies, we identified a highly active and reparative keratin. By protein sequence design, we engineered a water-soluble, thermally-stable, and structurally-optimized recombinant keratin with improved cost-effectiveness and enhanced biological performance, expanding its clinical applications.
Achieving Large-scale Production
Utilizing E. coli expression and a robust quality management system, we achieved large-scale production of high-purity recombinant keratin. The unique freeze-drying process preserves the product’s activity, stability, and safety, greatly lowering production, storage, and shipping costs.
Transforming Technologies into Commercial Use
The recombinant keratin technology has undergone successful technology transfer, enabling its integration into medical and consumer product lines.
Address
D-3-3, Encorp Strand Garden Office, No 12, Jalan PJU 5/1, Kota Damansara, 47810 Petaling Jaya, Selangor.
Contact
Phone +603-48183309
cs@shininghope.com.my
